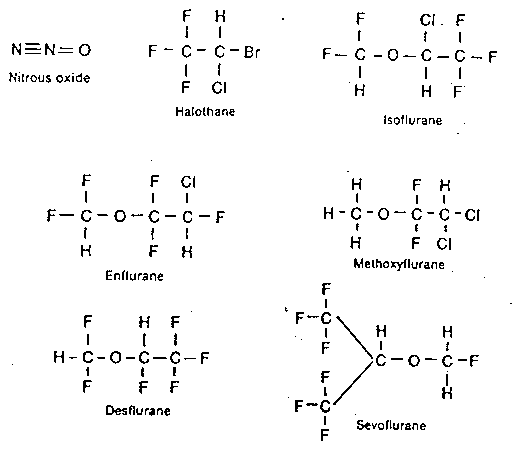
INHALATIONAL ANESTHETICS
Nitrous oxide
Only inorganic anesthetic gas in clinical use.
MAC: 105
Vapor pressure: --------
Blood/Gas Solubilities: 0.47
Characteristics:
Colorless and essentially odorless
Non-explosive and nonflammable, is as capable as oxygen of
supporting combustion.
Gas at room temperature and ambient pressure
Systemic Effects:
CNS:
Increases cerebral blood flow, producing mild elevation of
intracranial pressure.
Increases cerebral oxygen consumption (CMRO2).
Levels below MAC provide analgesia in dental surgery and other
minor procedures.
CVS:
Stimulates the sympathetic nervous system
Arterial BP, CO, HR are essentially unchanged or slightly
elevated because of stimulation of
catecholamines
Myocardial depression may be unmasked in patients with CAD or
severe hypovolemia. The
resulting decrease in arterial BP may occasionally lead to
myocardial ischemia.
Constriction of pulmonary vascular smooth muscle increases
pulmonary vascular resistance
®
of right atrial pressure
Peripheral vascular resistance is not significantly altered.
Nitrous oxide increases endogenous catecholamine levels, may
be associated with a higher
incidence of epinephrine-induced dysrhythmias.
MSK:
Does not provide significant muscle relaxation
Probably not a triggering agent of MH.
GI tract:
Implicated in postoperative nausea and vomiting presumably
as result of activation of the
chemoreceptor trigger zone and vomiting center in the
medulla.
Respiratory:
Increases respiratory rate (tachypnea) and decreases TV as a
result of CNS stimulation and
perhaps activation of pulmonary stretch receptors.
Net effect – minimal change in minute ventilation and
resting arterial CO2 levels.
Hypoxic drive is markedly depressed by even small amounts of
nitrous oxide.
Uterus:
Metabolism/Elimination:
Appears to decrease renal blood flow by increasing renal
vascular resistance ® a drop in GFR and
urinary output.
Hepatic blood flow probably falls but to a lesser extent than
with other volatile agents.
During emergence almost all nitrous oxide is eliminated by
exhalation. Small amount diffuses
through skin.
Bio-transformation is limited to the less than 0.01% that
undergoes reductive metabolism in the GI
tract by anaerobic bacteria.
Toxicity:
Prolonged exposure to anesthetic concentrations of nitrous
oxide can result in bone marrow
depression (megaloblastic anemia) and neurologic
deficiencies.
Because of possible teratogenic effects, it is often avoided
in pregnant patients.
May also alter immunologic responses to infection.
Contraindications:
It is 35 more times soluble than nitrogen in blood, tends
to diffuse into air-containing cavities
More rapidly than nitrogen is absorbed by the bloodstream.
Avoid with conditions of: air embolus, pneumothorax, acute
intestinal obstruction, intracranaial
air (tension pneumoencephalus following dural closur or
pneumoencephalography),
pulmonary air cysts, intraocular air bubbles, and tympanic
membrane grafting.
Will even diffuse into ET tube cuffs, increasing pressure
against tracheal mucosa.
Should be avoided with patients with pulmonary hypertension
It is of limited value in patients requiring high inspired
oxygen concentrations.
Drug interactions:
Because of its relatively high MAC, it prevents it use as
a complete general anesthetic. It is
frequently used in combination with more potent volatile
agents. The addition of nitrous oxide
decreases the requirements of these other agents.
It does attenuate the circulatory and respiratory effects of
volatile anesthestics in adults.
Potentiates neuromuscular blockade, but less so than the
volatile agents.
The concentration of N2O flowing through a vaporizer can
influence the concentration of volatile
anesthetic delivered. Example: Decreasing N2O concentration
(increasing oxygen
concentration) increases the concentration of volatile agent
despite a constant vaporizer
setting. This is due to the relative solubilities of N2O and
oxygen in liquid volatile anesthetics
(the second gas effect).
Miscellaneous:
Blue tank
750 psig until all liquid vaporized, then pressure drops in tank
Halothane (Fluthane) 1956
Halogentated alkane
MAC: 0.75
Vapor pressure: 243
Blood/Gas Solubilities: 2.4
Fat/blood Coefficient: 60
Characteristics:
Carbon-fluoride bonds responsible for its nonflammable and nonexpolsive
nature.
Stored in amber/brown bottle
Sweet, pleasant odor
Clear, colorless
Systemic Effects:
CNS:
Dilates cerebral vessels, which lowers cerebral vascular
resistance and increases cerebral blood
flow. Autoregulation, maintainence of constant cerebral blood
flow during changes in
arterial pressure, is blunted.
Concomitant rises in intracranial pressure can be prevented by
establishing hyperventilation prior
to halothane administration. Cerebral activity is decreased
leading to modest reductions in
metabolic oxygen requirements.
CVS:
Dose dependent reduction of arterial blood pressure is due
to direct myocardial depression;
2.0 MAC of halothane results in 50% decrease of BP and CO.
Cardiac interference with
intracellular calcium utilization , causes an increase in
right atrial pressure. Coronary blood
flow decreases due to the drop in systemic arterial pressure.
Adequate myocardial perfusion is
usually maintained since oxygen demand also drops. Halothane
blunts baroreceptor response.
Slowing of SA node conduction may result in junctional rhythm
or bradycardia.
Halothane prolongs QT interval (like other volatile agents).
Sensitizes the heart to the dysrhythmogenic effects of
epinephrine, so doses of epinephrine above
1.5 m g/kg should be avoided. May
result in halothane interfering with slow calcium
channel conductance.
Systemic vascular resistance is unchanged.
MSK:
Relaxes skeletal muscles and potentiates non-depolarizing
neuromuscular blocking drugs.
It is a triggering agent of MH.
GI tract:
Respiratory:
Causes rapid, shallow breathing. The increased respiratory
rate is not enough to counter the
decreased TV, so alveolar ventilation drops and resting PaCO2
is elevated. Apneic threshold,
the highest PaCO2 at which a patient remains apneic, also
rises because the difference between
it and resting PaCO2 is not altered by general anesthesia.
Halothane limits the increase in minute ventilation that
normally accompanies a rise in PaCO2.
Halothane’s ventilatory effects are probably due to central
(medullary depression) and peripheral
(intercostal muscle dysfunction) mechanisms. These are
exaggerated by preexisting lung
disease and attenuated by surgical stimulation.
The increase in PaCO2 and the decrease in intrathoracic
pressure that accompany spontaneous
ventilation with halothane partially reverse the CO, arterial
BP, and HR depression.
Hypoxic drive is severely depressed by even low concentrations
of halothane (0.1 MAC).
Potent bronchodilator, often reverses asthma-induced
bronchospasm. This is not inhibited by
propranolol (b -adrenergic
blocking agent).
Depresses clearance of mucous from the respiratory tract,
promoting postoperative hypoxia
and atelectasis.
Hepatic:
Causes hepatic blood flow to decrease in proportion to the
depression of cardiac output.
Could have minor liver transaminase elevations.
Uterus:
Profound effect. Inhibits myo-uterine tone – profound
bleeding.
Only use if need to relax uterus.
Metabolism/Elimination:
Metabolism and clearance of some drugs (eg, fentanyl,
phenytoin, verapamil) appeared to be
impaired by halothane.
Oxidized in the liver to its principal metabolite,
trifluoroacetic acid.
Bio-transformation in liver – cP450 system.
Toxicity:
Halothane hepatitis –extremely rare (1 per 35,000
cases). Patients exposed to multiple halothane
anesthetics at short intervals, middle-aged obese women, and
persons with a familial
presdisposition to halothane toxcity or personal history are
considered at increased risk.
Centrilobular necrosis – implies hepatic damage from
reductive metabolites or hypoxia. Could
also be related to an immune mechanism. This has implicated
liver microsomal proteins
that have been modified by trifluoroacetic acid as the
triggering antigens.
Contraindications:
Withhold halothane from patients with unexplained liver
dysfunction following previous
exposure. Halothane hepatitis appears to affect primarily
adults and children past puberty.
Should be used with caution in patients with intracranial mass
lesions because of the possibility
of intracranial hypertension.
Hypovolemic patients and some patients with severe cardiac
disease (aortic stenosis) may not
tolerate halothanes’s negative inotropic effects.
Sensitization of the heart to catecholamines limits the
usefulness of halothane when exogenous
epinephrine is administered or in patients with
pheochromocytoma.
Drug interactions:
Myocardial depression seen with halothane is exacerbated by b
-adrenergic blocking agents
(eg, propranolol) and calcium channel-blockig agents (eg,
verapamil).
Tricyclic antidepressants and MAO inhibitors have been
associated with BP fluctuations and
dysrhythmias, although neither represents fluctuations an
absolute contraindication.
Combination of halothane and aminophylline has resulted in
serious ventricular dysrhythmias.
Enflurane (Ethrane)
Halogenated ether.
MAC: 1.7
Vapor pressure: 175
Blood/Gas Solubilities: 1.9
Characteristics:
Mild, sweet, ethereal odor and is nonflammable at clinical concentrations.
Systemic Effects:
CNS:
Increases cerebral blood flow and intracranial pressure.
Has been shown to increase the secretion of CSF and the
resistance to CSF outflow.
Hyperventilation is not recommended to attenuate enflurane-induced
intracranial hypertension.
Deep enflurane anesthesia can culminate in frank tonic-clonic
seizures.
Cerebral metabolic requirements are decreased by enflurane
unless seizure activity is initiated.
CVS:
Depresses myocardial contractility. This negative
inotropic action appears to involve depression
of calcium influx and SR release during myocardial membrane
depolarization.
Arterial BP, CO, and myocardial oxygen consumption are
lowered.
Decreases SVR; HR usually rises.
Sensitizes the heart to the dysrhythmic effects of
epinephrine, but doses up to 4.5 m g/kg are
usually well tolerated.
MSK:
Relaxes skeletal muscle.
Decrease amount of non-depolarizing muscle relaxant (1/3 to
˝). Enhance and prolong effects.
Respiratory:
Decrease minute ventilation despite an increase in RR and
increased resting PaCO2, decreased
response to hypercapnia, abolishment of hypoxic drive,
depressed mucociliary function,
and bronchodilation.
GI tract:
Hepatic:
Decrease in hepatic blood flow is similar to that caused
by equipotent doses of other volatile
agents.
Uterus:
Metabolism/Elimination:
RBF, GFR, and urinary output fall during enflurane
anesthesia. A metabolite of enflurane is
nephrotoxic.
Metabolized in liver. Biotranformation - microsomal
Toxicity:
Contraindications:
Should be avoided in patients with pre-existing disease
even though deterioration in renal function
is unlikely.
Should also choose another agent for patients with seizure
disorders.
Precautions concerning intracranial hypertension, hemodynmic
instability, and MH are the same
as those associated with halothane.
Drug interactions:
Isoniazid induces enflurane defluorination. May be
clinically significant in so-called rapid
Acetylators (patients with an autosomal trait that increases
the rate of hepatic acetylation).
Isoflurane (Forane) 1981
Chemical isomer of enflurane, it has different physiochemical properties.
MAC: 1.2
Vapor pressure: 240
Blood/Gas Solubilities: 1.4
Characteristics: Nonflammable, pungent ethereal odor.
Systemic Effects:
CNS:
At concentrations greater than 1 MAC, increases cerebral
blood flow and intracranial pressure.
The effects are thought to be less pronounced than with
halothane or enflurane and are reversed
by hyperventilation. The hyperventlation does not have to be
instituted prior to the use of
isoflurane to prevent intracranial hypertension (unlike
halothane).
It reduces cerebral metabolic oxygen requirements and at 2 MAC
produces an electrically silent
EEG.
CVS:
Causes minimal cardiac depression. CO is maintained by a
rise in HR due to partial preservation
of carotid baroreflexes.
Mild b -adrenergic stimulation
increases skeletal muscle flow, decreases SVR, and lowers
arterial BP.
Rapid increases in isoflurane concentration lead to increases
in HR, arterial BP, and plasma
levels of norepinephrine.
Dilates coronary arteries (discussion of coronary steal
syndrome). Usually avoided in patients
with coronary artery disease.
MSK:
Relaxes skeletal muscle.
Respiratory:
Respiratory depression resembles that of other volatile
anesthetics, except that tachypnea is less
pronounced. The net effect is a more pronounced fall in
minute ventilation.
Low levels of isoflurane (0.1 MAC) blunt the normal
ventilatory response to hypoxia and
hypercapnia.
Considered a good bronchodilator.
GI tract:
Hepatic:
Total hepatic blood flow is reduced. Hepatic oxygen supply
may be better maintained with
isoflurane because hepatic artery perfusion is maintained.
Liver function tests are minimally affected.
Uterus: In high doses – uterine relaxation.
Metabolism/Elimination:
Metabolized to 1/10 of the extent of enflurane.
Trifluoroacetic acid is the principal end product.
Nephrotoxcity is unlikely.
Toxicity:
Contraindications:
Presents no unique contraindications other than the
controversy of possible coronary steal.
Patients with severe hypovolemia may not tolerate its
vasodilating effects.
Drug interactions:
Epinephrine can be safely administered in doses up to 4.5 m
g/kg.
Non-depolarizing muscle relaxants are potentiated by
isoflurane.
Desflurane (Suprane) 1992
Structure is very similar to that of isoflurane. Only difference is a
substitution of a fluorine atom for isoflurane’s chlorine atom. Has profound
effects on physical properties of drug.
MAC: 6.0
Vapor pressure: 681 (boils at room temperature)
Blood/Gas Solubilities: 0.42 Rapid wash-in and wash-out of anesthestic.
Alveolar concentration will tend
to approach inspired concentrations much more rapidly than
other volatile agents, giving tighter
control over anesthetic level. Wake up times are approximately
half as long as those observed
with isoflurane.
Characteristics:
High vapor pressure, ultra-short duration of action, and
moderate potency – most characteristic
features.
Special vaporizer needed.
Systemic Effects:
CNS:
Decreases cerebral vascular resistance, increases cerebral
blood flow, and is associated with an
increase in ICP at normotension and normocapnia.
Cerebral vasculature remains responsive to changes in PaCo2,
so that intracranial pressure can be
lowered by hyperventilation.
Cerebral oxygen consumption is decreased. During periods of
desflurane induced hypotension
MAP=60 mmHg), cerebral blood flow is adequate to maintain
aerobic metabolism despite a
low cerebral perfusion pressure.
CVS:
Similar to those of isoflurane. Increasing the dose is
associated with a decline in SVR that leads
to a fall in arterial BP.
CO remains relatively unchanged or slightly depressed at 1-2
MAC.
There is a moderate rise in HR, CVP, and pulmonary artery
pressure that may not be apparent at
low doses.
Rapid increases in desflurane concentration lead to transient
elevations in HR, BP, and
catecholamine levels more pronounced than isoflurane.
Does not increase coronary blood flow.
MSK:
Associated with a dose-dependent decrease in the response
to train-of-four and tetanic
peripheral nerve stimulation.
Hepatic:
Hepatic function tests are unaffected.
Respiratory:
Causes a decrease in TV and an increase in respiratory
rate.
There is an overall decrease in alveolar ventilation that
causes a rise in resting PaCO2.
Depresses the ventilatory response to increasing PaCO2.
Pungency and airway irritation during induction can be
manifested by salivation, breath-holding
coughing, and laryngospasm.
GI tract:
Uterus:
Metabolism/Elimination:
No evidence of any nephrotoxic effects.
Undergoes minimal metabolism in humans.
Toxicity:
Contraindications:
Shares many of the contraindications of other modern
volatile anesthetics: severe hypovolemia,
MH, and intracranial hypertension.
Drug interactions:
Poteniates non-depolarizing muscle relaxants to the same
extent as isoflurane.
Epinephrine can be safely administered in doses up to 4.5 m
g/kg – desflurane does not sensitize
the myocardium to the dysrhythmogenic effects of epinephrine.
Sevoflurane (Ultane) 1995
Halogenated with fluorine.
MAC: 2.0
Vapor pressure: 160
Blood/Gas Solubilities: 0.65
Characteristics:
Potency slighty less than enflurane.
Nonpungency and rapid increases in alveolar concentration make sevoflurane an
excellent choice for
inhalational inductions.
Systemic Effects:
CNS:
Causes slight increases in cerebral blood flow an
intracranial pressure at normocarbia.
Cerebral metabolic oxygen requirements decrease, and no
seizure activity has been reported.
CVS:
Mildly depresses myocardial contractility. SVR and
arterial BP decline slightly less than with
isoflurane or desflurane.
Because of little if any rise in HR, CO is not well maintained
as with isoflurane or desflurane.
No evidence associating it with coronary steal syndrome.
MSK:
Produces adequate muscle relaxation for intubation of
children following an inhalational
induction.
Respiratory:
Depresses respiration and reverses bronchospasm to an
extent similar to isoflurane.
GI tract:
Hepatic:
Decreases portal vein blood flow, but increases hepatic
artery blood flow, maintaining total
hepatic blood flow and oxygen delivery.
Uterus:
Metabolism/Elimination:
Liver microsomal enzyme P450 metabolizes sevoflurane at a
rate similar to enflurane and may be
induced with ethanol or phenobarbital pretreatment.
Decreases renal blood flow – its metabolism to fluoride has
been associated with impaired renal
tubule function. There is potential nephrotoxicity of the
resulting rise in inorganic fluoride (F-).
Should be avoided in patients with impaired kidney function.
Toxicity:
Alkali such as soda lime can degrade sevoflurane,
producing nephrotoxic end product (compound
A, an olefin). Accumulation of compound A increases with
increased respiratory gas
temperature,low-flow anesthesia, dry barium hydroxide
absorbent, high sevoflurane
concentrations, and anesthetics of long duration.
Determination of whether sevoflurane
anesthesia achieves toxic concentrations of compound A is yet
to be determined. Fresh gas
flows less than 2 liters/min are not recommended.
Contraindications:
Include severe hypovolemia, susceptibility to MH, and
intracranial hypertension.
Drug interactions:
Potentiates non-depolarizing muscle relaxants. It does not
sensitize heart to catecholamine-
-induced dysrhythmias.
Molecular Structures of Inhaled Anesthetics

Last Updated 09/06/01 08:53:10 PM
Return To MNA2001 Homepage