Guyton, Chapter 19
Ganong, Chapter 31
renal/body fluid mechanism for long-term blood pressure control: When body contains too much ECF, the arterial pressure increases. The rising pressure has a direct effect to the kidneys to excrete the excess ECF, returning the pressure back to normal. The increase in arterial pressure of a few millimeters can double the renal output of water. This is further refined with the Renin-Angiotensin mechanism. (Guyton, p. 221)
These involve the renal output of water and sodium. To increase the water output there will be an increase in the pressure, which will increase the GFR, which will drive more water through the filter and thus decrease total body water. This is called pressure diuresis. The same thing can be said of sodium, which is called pressure natriuresis. The third factor has two sections – one is the release of ANP, which increases sodium excretion, and the second is Endogenous-digitalis-like substance (EDLS) which decreases the Na/K/ATPase pump activity so when the pump is inhibited, more sodium will be excreted in the urine. An increase in sodium excretion will decrease body fluid and thus decrease blood pressure.
chronic renal function curve: Represents everything (hormonal activity) operating in a healthy human subject. (Class notes – 10/7)

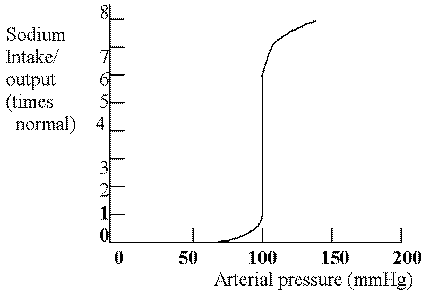
The average effect of different arterial pressures on urine volume output is marked increase in output of volume as pressure rises (pressure diuresis), and also about an equal effect on sodium output (pressure natruresis). (Guyton, p. 221-222)
This curve demonstrates that different arterial pressures affect urine output by increasing the output volume as the pressure rises. This is known as pressure diuresis. Increasing the pressure will also increase the sodium excretion, which is called pressure natriuresis.
pressure natriuresis: Increase in arterial pressure of a few millimeters of Hg that can double the renal output of salt. (Guyton, p. 221)
To increase blood pressure will increase the GFR due to a change in Starling’s forces of capillary exchange such as oncotic pressure, hydrostatic pressure and the filtration coefficient. This in turn will increase the amt of sodium that is filtered and excreted.
atrial natriuretic peptide (ANP): Secreted by the heart, antagonizes the action of various vasoconstrictor agents an lowers blood pressure (Ganong, p. 572)
Secreted by the atria in response to increases in ECF volume and atrial pressure. Its most important action is relaxation of vascular smooth muscle, which results in vasodilation and decreased TPR. In the kidney , this vasodilation leads to increased Na+ and water excretion, thereby decreasing ECF volume and blood volume, and decreasing Pa. (Costanzo, p. 146)
This substance is released by the atria when an excess fluid volume stretches the atria and BP is elevated. ANP affects the RAA system and is the physiological antagonist to the RAA system. ANP caused an increase in sodium excretion and therefore a decrease in ECF, which will decrease MSAP.
natriuretic hormone: Hormone that promotes excretion of sodium.
Another name for ANP and EDLS
renin-angiotensin-aldosterone system: Regulates Pa primarily by regulating blood volume. It is much slower than the baroreceptor reflex, because it is hormonally, rather than neurally, mediated. It is activated in response to a decrease in the Pa. Activation produces a series of responses that attempt to restore arterial pressure to normal.

Renin is released by the juxtoglomerular cells in afferent arterioles of the kidney in response to SNS stimulation. The receptors that mediate this are beta receptors on cells. Renin will then increase the production of angiotensin I which will lead to Angiotensin II which is a potent vasoconstrictor which then increases total peripheral resistance. Angiotensin II will also stimulate the release of aldosterone from the medulla which will increase sodium reabsorption so less Na leaves the body and more stays in which increase ECF volume. ADH or vasopressin is also stimulated when there is a low MSAP to hold on to water in the renal tubules and thus increase ECF volume.
chronic hypertension: High blood pressure – when the mean arterial pressure is greater than 110mmHg under resting conditions. At this level the diastolic blood pressure is greater than 90mmHig and the systolic BP is greater than 135-140mmHg.
pressure overload: Hypertension caused by an excess accumulation of ECF fluid in the body. This also refers to the increase in afterload leading to chronic HTN and ventricular hypertrophy.
ventricular hypertrophy: Enlargement of the ventricle wall, due to increase preload (volume) – eccentric enlargement, or increased afterload (pressure) – concentric enlargement. (Class notes – 10/7)
The structural remodeling that occurs from chronic changes in pressure or tension in the wall of the ventricle resulting in a larger than normal heart with wall thickening of the ventricle. In concentric hypertrophy the remodeling is a result of a chronic increase in afterload due to an increase in MSAP. The ratio between wall thickness and pressure will normalize the wall tension. It will reduce the tension per square surface area since there is more surface area to spread out the tension thus normalizing the tension. The radius often decreases as a result. Eccentric hypertrophy is due to a chronic increase in preload. There is a stronger than normal force of contraction since the filling pressures are greater and so the SV is greater. Starling’s Law states that the greater the tension or stretch the greater the contraction. Therefore wall tension is chronically increased and this results in remodeling of the ventricular wall again but this time the CXR shape is elongated and off center. This thickness is also associated with an increase in radius to keep their ratio equal.
peripheral vascular autoregulation: Maintenance of constant blood flow. Ex: arterial circulation pressure is decreased ® vasodilation ® decreased resistance – keeping the flow constant.
The peripheral blood vessels will return their blood flow back to normal after a sudden increase within less than a minute. There is the metabolic theory that states when the art pressure becomes too great, there is an excess flow of oxygen and nutrients which causes the blood vessels to constrict and flow to return to normal and there is the myogenic theory that states the sudden stretch of small blood vessels cause the smooth muscle of the vessel wall to contract and this reduces the blood flow.
peripheral resistance: Impedence of blood flow in vessels – systemic circulation (arteries/veins).
This is the impediment to blood flow in a vessel. Sources of resistance are the geometry of the vessels – the physical properties of the vessels such as radius and length, bifurcations, obstructions, valves and the viscosity of the blood.
hypertensive vascular disease: Chronic HTN in the vessels. Remodeling occurs in response to the chronic high pressure.
sodium balance: Sodium balance in body affected by factors:
A long-term regulation feature of the kidneys in the maintenance of normal blood pressure. Different hormones control the amt of sodium excreted or reabsorbed in the renal tubules thus controlling urine output and total ECF volume.
stress: Environmental source - SNS ® TPR (Class notes 10/7)
This can be acute or chronic and the body will adjust accordingly. Acute stress is sensed by the adrenal glands, which then secrete epi and norepi, which will cause increase HR, contractility, peripheral vasoconstriction and skeletal muscle vasodilation. Chronic stress is also reacted to by the adrenal glands and a steady state of hormone production but there can be fatigue of the glands or the vessels/heart remodel to adapt to the new stress of high pressures for example.
volume-loading hypertension: Means hypertension caused by an excess accumulation of ECF fluid in the body. This also refers to the increase in afterload leading to chronic HTN and ventricular hypertrophy.
Hypertension
caused by excess accumulation of ECF in the body.
Example:
Have decreased kidney mass.
Intake of salt/water increased to about 6x normal.
Acute effect – is the increase in ECF volume, blood volume and CO to 20 to 40
% above normal.
Simultaneously arterial pressure begins to rise but not as fast as the fluid
volumes and CO.
There is initial decrease in TPR by the baroreceptors mechanism (tries to prevent the rise in pressure). After a few days the baroreceptors adapt (reset) and no longer oppose the rise in pressure. Arterial pressure has now risen almost to its full height because of the increased CO, even though TPR is almost at normal level.
More prolonged secondary changes occur during the next few days and weeks.
There is progressive increase in TPR while at the same time the CO is decreased back to normal. (Changes almost certainly caused by the long-term blood flow autoregulation mechanism).
CO has risen to high level and had initiated the hypertension, the excess blood flow through the tissues than caused progressive constriction of the local arterioles, thus returning the local blood flow and the CO almost back to normal, but simultaneously causing a secondary increase in TPR.
The increased TPR occurs after the hypertension has developed and therefore is secondary to the hypertension, rather than the cause of it.
Clinical examples:
essential hypertension: Hypertension of unknown origin. 90% of people with hypertension are said to have this. It is treatable but not curable. There a strong hereditary tendency.
Characteristics of severe essential hypertension:
(Guyton, p.233-234)
describe using words and graphs or diagrams the LONG-TERM HIGH-GAIN RENAL/BODY FLUID MECHANISM for controlling mean systemic arterial pressure through the regulation of SODIUM EXCRETION and extracellular fluid volume: A high gain control system can make large corrections with a small residual error. A long-term system gives the overall system longer to achieve this steady state. Sodium excretion will occur for several reasons if the MSAP is elevated. 1st there is the increase in GFR as a result of the higher pressure. The higher filtration rate will increase the filtration of sodium and more will be excreted in the urine. This is called pressure natriuresis. 2nd there is a decrease secretion of renin by the kidneys when MSAP is elevated. The decrease in the RAA will have an inhibitory effect on the reabsorption of sodium so more will be excreted. 3rd there is an increase in the release of ANP and EDLS when the atria are stretched by an excess in ECF volume. ANP will cause the kidneys to excrete more sodium and antagonizes the RAA system. EDLS will inhibit the Na/K/ATPase pump in the renal tubules thus inhibiting the ability to pump the Na back into the plasma and this allows more to then be excreted. All of these systems affect the kidneys to excrete more sodium thus decreasing ECF volume and this will then decrease MSAP.
on a graph relating mean arterial pressure (abscissa) and the rate of steady-state sodium excretion or intake (ordinate), draw the CHRONIC RENAL FUNCTION CURVE and explain its significance in LONG-TERM BLOOD PRESSURE CONTROL: (See Graph Above) The chronic renal function curve takes into account several control mechanisms. The ANP system is activated when there is too much fluid in the ECF and this inhibits the RAA system and causes the kidneys to excrete more sodium thus decreasing ECF and decreasing the MSAP back to normal. The RAA system is activated when there is too little fluid in the ECF or the MSAP is low. Renin converts to angiotensin II which is a potent vasoconstrictor so TPR increases which increases MSAP. Angiotensin also affects the kidneys to reabsorb sodium, which will increase the ECF and thus increase MSAP. Angiotensin also stimulates the secretion of aldosterone, which increases water reabsorption in the renal tubules and this increase the ECF volume, which will increase BP. The renal function curve has an infinite gain to it and is almost a straight line because it will readjust the sodium intake and fluid volume status to maintain MSAP at a steady state. It may take several days to achieve this effect but it will do it with a minimal residual error.
compare and contrast what is known about the structures, site(s) of origin, and postulated mechanism(s) of action of ATRIAL NATRIURETIC PEPTIDE (ANP) and ENDOGENOUS DIGOXIN-LIKE SUBSTANCE (NATRIURETIC HORMONE)
Atrial Natriuretic Peptide (ANP)
This is secreted by the atria in response to increases in ECF volume and
atrial pressure. ANP has multiple effects, but its most important action is
relaxation of vascular smooth muscle, which results in vasodilation and
decreased TPR. Vasodilation leads to increased Na+ and water
excretion, thereby decreasing total body Na+ content, decreasing
ECF volume and blood volume, and decreasing Pa. (Costanza, p. 146)
The muscle cells in the atria contain secretory granules that increase in number when sodium chloride intake is increased and ECF expanded, and extracts atrial tissue cause natriuresis.. It is a polypeptide with a characteristic 17-amino-acid ring formed by a disulfide bond between two cysteines. The circulating form has 28 amino acid residues. It is formed from a large precursor molecule that contains 151 amino acid residues, including a 24-amino-acid signal peptide. ANP causes natriuresis, which may be due to an increase in GFR. There are ANP receptors on the mesangial cells in the glomeruli, and the relaxation of these cells produced by ANP presumably increases the effective surface area available for filtration. ANP could act on the tubules to promote sodium excretion. The exact mechanism is unknown. ANP also lowers blood pressure, decreases the responsiveness of vascular smooth muscle to many vasoconstrictor substances, decreases the responsiveness of the zona glomerulosa to stimuli that normally increase aldosterone secretion, and inhibits the secretion of vasopressin. ANP inhibits renin secretion and consequently lowers circulating angiotensin II. (Ganong, p. 439-440)
Endogenous Diogoxin-Like Substance (Natriuretic Hormone) - EDLS
Hormone produced in hypothalmus. It is not a protein or peptide. It
increases the Ca2+ concentration in smooth muscles for contraction.
It is like digitalis – decreases the activity of Na+-K+
ATPase, decreasing Na+ reabsorption (
Na+ excretion). EDLS will also increase TPR ®
increased MSAP.
ANP comes from the cells of the atria. It is an 8-chain peptide that promotes sodium excretion in the kidneys. When the CVP is elevated the atria are stretched which causes them to secrete ANP and this leads to a natriuresis. ANP is the physiological antagonist to the RAA system. This is a link between the cardiovascular system and the renal body fluid system – a link of short-term stabilization and long-term changes of the set point. EDLS is produced in the hypothalamus. It is not a protein or a peptide. When volume is expanded the atria send a signal to the hypothalamus and EDLS is released. It acts like a natriuretic and allows sodium to be excreted. EDLS acts by inhibiting the Na/K/ATPase pump thus impairing the amt of sodium reabsorbed into the renal tubules, which is dependent on this pump to reabsorb sodium. Sodium can then rely on the cation exchanger to move out of the tubules but this moves calcium in and calcium moving in will cause and increase in contraction. This can increase TPR which can further increase MSAP thus could lead to HTN.
describe using words and diagrams a theory for the PATHOGENESIS OF HYPERTENSION which incorporates genetic predisposition(s), dietary sodium intake, STRESS, atrial natriuretic factor, and natriuretic hormone: EDLS acts by inhibiting the Na/K/ATPase pump thus impairing the amt of sodium reabsorbed into the renal tubules, which is dependent on this pump to reabsorb sodium. Sodium can then rely on the cation exchanger to move out of the tubules but this moves calcium in and calcium moving in will cause and increase in contraction. This can increase TPR which can further increase MSAP thus could lead to HTN. Genetically some people are predisposed to developing HTN. If these people were to have an impairment in one control system or another or they were to increase their dietary sodium intake, this could lead to HTN in them. Dietary sodium intake that is elevated will set off the renal body fluid mechanisms for fluid control to maintain MSAP. Chronically high sodium levels can eventually change the set point for normal MSAP and can lead to HTN. Stress causes the SNS to release epi and norepi, which cause vasoconstriction and thus increase MSAP so chronic stress can cause a chronic elevation in MSAP, which will lead to HTN. ANP should antagonize the RAA system. Chronic elevated release of the RAA system would lead to HTN but ANP should counteract this so ANP would help you maintain normal BP.
describe the relationships among age, sex, race, diet, and obesity and the incidence and severity of hypertension: Older women, men, African-American people, high fat, salt diets and obese people are more at risk for developing HTN.
discuss the role of AUTOREGULATORY COMPENSATORY MECHANISMS in the peripheral circulation in the PATHOGENESIS OF ELEVATED PERIPHERAL VASCULAR RESISTANCE in established hypertension: Chronic high pressures will cause the release of endothelin which will vasoconstrict the capillary walls to limit the blood flow to match demand of nutrients. Endothelin will lead to the remodeling of the vascular wall to thicken with elastin and collagen. This narrows the radius also, which physically leads to lower pressures through this area. The TPR is higher but the newly modeled vessels now require this higher resistance in order to meet flow requirements.
discuss using words and diagrams the LONG-TERM STRUCTURAL CONSEQUENCES of chronic hypertension on the heart and peripheral vasculature: Chronic HTN causes hypertrophy of the heart and vessels, which leads to a diminished capability to respond to large changes in volume status. The hypertrophied areas are stiffer and can’t contract as well with lower volumes and require higher volumes to maintain normal cardiac outputs. The fine line of control can be easily crossed and one can fall over the line into CHF due to the hypertrophy and decreased capabilities to maintain BP control.
describe the role of the renin-angiotensin-aldosterone system in sodium homeostasis and the rationale for pharmacological manipulation of this system in patients with hypertension: Angiotensin II is blamed for the remodeling that occurs in myocardial and vascular cells thus leading to hypertrophy. ACE inhibitors inhibit the angiotensin activity thus prevent this remodeling. They also inhibit the functions of angiotensin, which is to promote sodium reabsorption, which would lead to an increase in ECF volume and further HTN. So ACE inhibitors help decrease HTN associated with the RAA system.